What exactly are Redox Reactions?
A redox reaction is described as a chemical reaction where electrons are transferred between two reactions taking part in it. This technique of transferring electrons may be identified by observing modifications from the oxidation states from the reacting species.
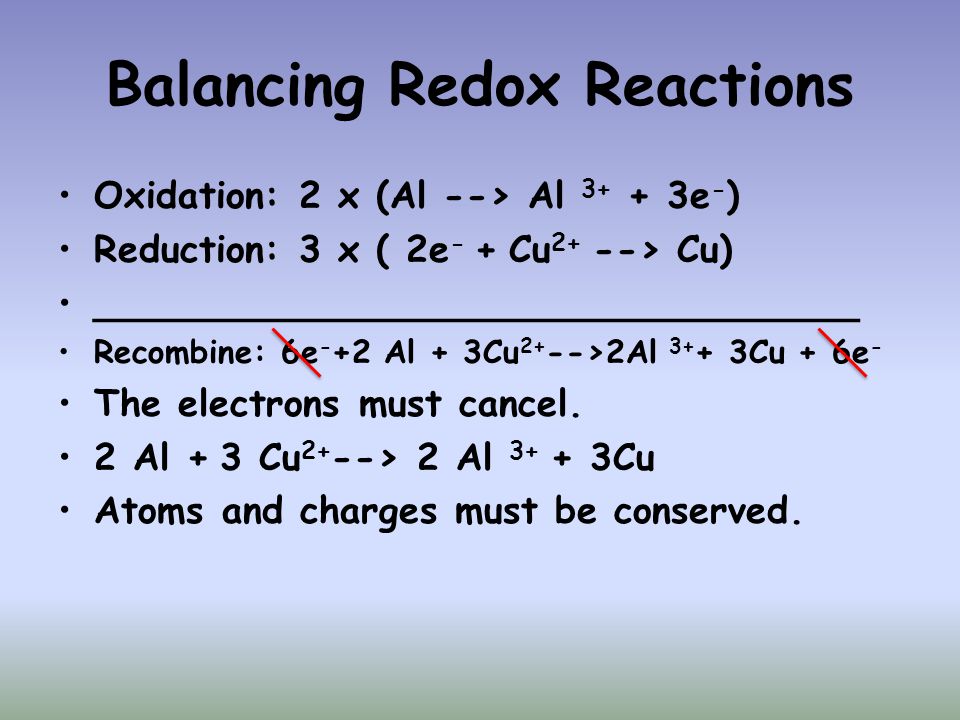
Losing electrons along with the corresponding boost in the oxidation condition of the reactant is known as oxidation. The gain of electrons and the corresponding decrease in the condition of the reactant is known as reduction.
The electron-accepting species that undergo a reduction in redox reactions these are known as oxidizing agents. The electron-donating species that tend to hand over electrons are called reducing agents.
Redox Reaction Calculator: Do you feel it really is complex to fix the redox reaction for the chemical reaction? If so, then utilise online handy calculator mainly because it does lengthy calculations and gives the exact answer in a fraction of seconds.
Steps to Calculate Redox Reaction
Below-mentioned will be the detail by detail process on how to solve the redox result of a compound reaction.
Receive the standard potential, the quantity of electrons transferred throughout the reaction, temperature, and ratio from the concentration of product versus reactant details.
Divide temperature by the variety of electrons transferred.
Find the log from the ratio from the energy product versus reactant value.
Multiply the division result from the log result value.
Subtract the obtained product from standard possibility to know the redox reaction value.
To get more information about Balance redox reactions see this website
